A covalent bond is formed by two atoms sharing a pair of electrons. The atoms are held together because the electron pair is attracted by both of the nuclei.
In the formation of a simple covalent bond, each atom supplies one electron to the bond - but for a co-ordinate bond (also called as dative bond) a covalent bond when a shared pair of electrons come from the same atom.
In a lewis structure, bond is shown by an arrow. The arrow points from the atom donating the lone pair to the atom accepting it.
Example:
Ammonium ion
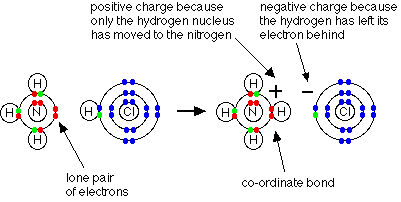
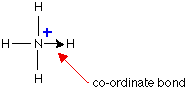
Example:
In the formation of a simple covalent bond, each atom supplies one electron to the bond - but for a co-ordinate bond (also called as dative bond) a covalent bond when a shared pair of electrons come from the same atom.
In a lewis structure, bond is shown by an arrow. The arrow points from the atom donating the lone pair to the atom accepting it.
Example:
Ammonium ion